|
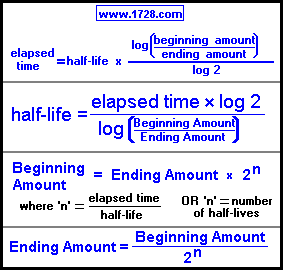
However, if you are just here to use the calculator then stay on this page.
elapsed time = 87.51 * (log (100 / 10) / .30103) elapsed time = 87.51 * (1 / .30103) elapsed time = 290.70 days
Half life = (120 * .30103) / log (1 / .54821) Half life = 36.1236 / log (1.8241) Half life = 36.1236 / .26105 Half life = 138.38 days 3) Your professor tells you to measure a sample of phosphorus-32 (half life = 14.263 days). You forget about this until 7 days later, you measure its mass to be 37 grams. (Luckily, in that intervening week, the sample was safely isolated and all safety rules and regulations regarding the proper handling of radioactive materials were strictly enforced.)  What was the mass of phosphorus-32 one week before, when you were supposed to measure it?
Beginning amount = 37 * 2(7 / 14.263) Beginning amount = 37 * 2(.49078) Beginning amount = 37 * 1.4052 Beginning amount = 51.993 grams 4) Hydrogen-3 or tritium as it is commonly called, has a half life of 12.32 years. If you start with 20 grams of it, how much will remain after 25 years?
Ending Amount = 20 / 2(25 / 12.32) Ending Amount = 20 / 2(2.0292) Ending Amount = 20 / 4.0818 Ending Amount = 4.8997 grams
Answers are displayed in scientific notation and for easier readability, numbers between .001 and 1,000 will be displayed in standard format (with the same number of significant figures.) The answers should display properly but there are a few browsers that will show no output whatsoever. If so, enter a zero in the box above. This eliminates all formatting but it is better than seeing no output at all.
Copyright © 1999 - 1728 Software Systems |
||||